Pharmira’s Continuous
Manufacturing Technology
What is “Continuous Manufacturing”?
Continuous Manufacturing is a manufacturing method in which raw materials or their blended materials are entered continuously in the manufacturing process throughout the duration of the process, and products are produced continuously through the manufacturing.
PMDA Views on Applying Continuous Manufacturing to Pharmaceutical Products for Industry (provisional draft) [PMDA 2018]
Continuous Manufacturing is combined process consisted of two or more continuous unit operations.
Continuous Manufacturing for Pharmaceutical Products
Necessary for ensuring that product made by a continuous manufacturing process is homogenous and of desired quality by control strategies.
Merits of Continuous Manufacturing
You can see it on the slide.
Changes of External Circumstance
Merit of Continuous Manufacturing of APIs

Rapid shortening for drug development
- Rapid screening/judgement
- Emergent permission related to COVID-19
Shortening development period
- Omitting scale-up
- Reduction of development cost
- Shortening R&D period
Cost reduction such as scale-up R&D, trial manufacture by batch, raw materials, stability testing, tech-transfer
Securing domestic supply chain of pharmaceutical products
- Safe domestic supply of antibacterial agent
- Reconsidering global supply chain
- Recent trend including US.
Flexible Manufacturing Organization
- Reduction of manufacturing cost
- Safe supply of APIs
- Flexible adjustment of production quantity
Enhancement of regulation for quality of pharmaceutical products
- ICH M7 (error-prone impurities)
- ICH Q3D (element impurities)
Accurate Quality Control & Assurance
- Online quality monitoring by PAT (Process Analytical Technology) tool and RTRT (Real Time Release Testing)
Risk avoidance by scale-up
Protect operators & environment
- Increase of APIs with high activity
- Energy saving, reduction of CO2 emission
Reduction of Environmental Load
- Automation and compact manufacturing line
- Reduction of waste by high efficient manufacturing
※1 ICH : International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
※2 PAT:Process analytical technology
※3 RTRT: Real time release testing
Comparison between continuous manufacturing and batch manufacturing
| batch manufacturing | continuous manufacturing | |
| Injection of raw materials / collection of products |
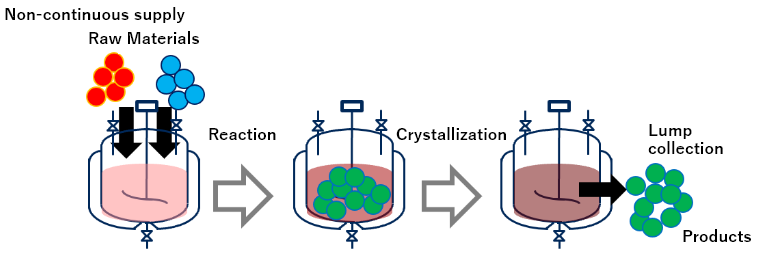 ■Inject raw material collectively or dividedly, means not continuously ■Products are collected all together after operation |
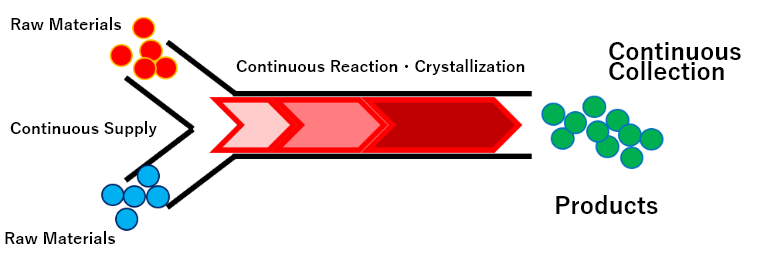 ■Inject raw material and/or blended one to the manufacturing process continuously ■Products are collected continuously |
| Intervention by operator | Yes (start/stop by each operation) | None (unit operation is connected and transferred to the next process automatically) |
| Quality control | Process parameter control, Process administration test, Real time release testing, Specification test etc | In addition to the mention left side, exclude defected material in defined period of time based on In-line test result |
| Scale-up | Verification is necessary in each scale | Enable to the effective study period considering with applying equipment in R&D period to commercial production scale (high-speed commercialization) |
| Footprint of manufacturing plant | Large | Small |
| Background | Comparing with basic chemical goods, non-necessity for large-scale production and high-profitability, therefore, batch manufacturing is enough in previous | Gradually accepted due to the needs for cutting cost and improved quality of pharmaceutical products |
| Production volume | Injection volume X number of batch | Speed for production X Time |
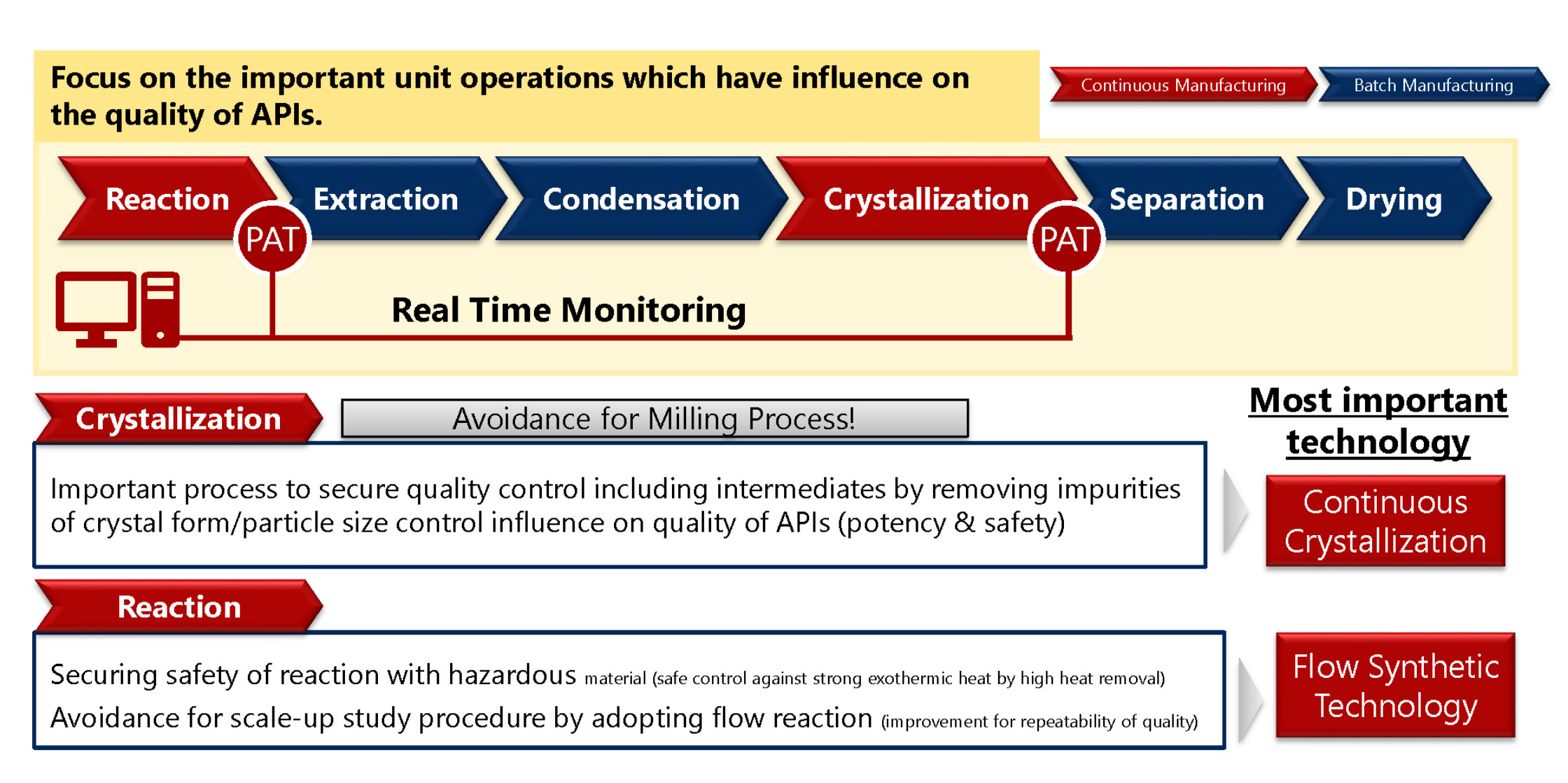
Feature of Pharmira’s Continuous Manufacturing
Focus on the important unit operations (reaction and crystallization) for the quality of APIs.
- Applying for photoreaction, special reaction, hydrogenation reaction etc which is presumably compatible to flow synthesis and continuous manufacturing
- Apply for continuous crystallization
ー Control crystal form and particle size, omitting milling process
ー High quality control with repeatability - Capacity : 0.02 – 0.1 kg/hour
Manufacturing Process with Continuous Manufacturing Technology
(Hybrid operation by batch and continuous technology)
Example of facilities and technologies

Flow Reaction Equipment

Flow synthetic process technology by photocatalyst

Continuous crystallization module
